Water-Soluble Palladium Reagents for Cysteine S-Arylation under Ambient Aqueous Conditions
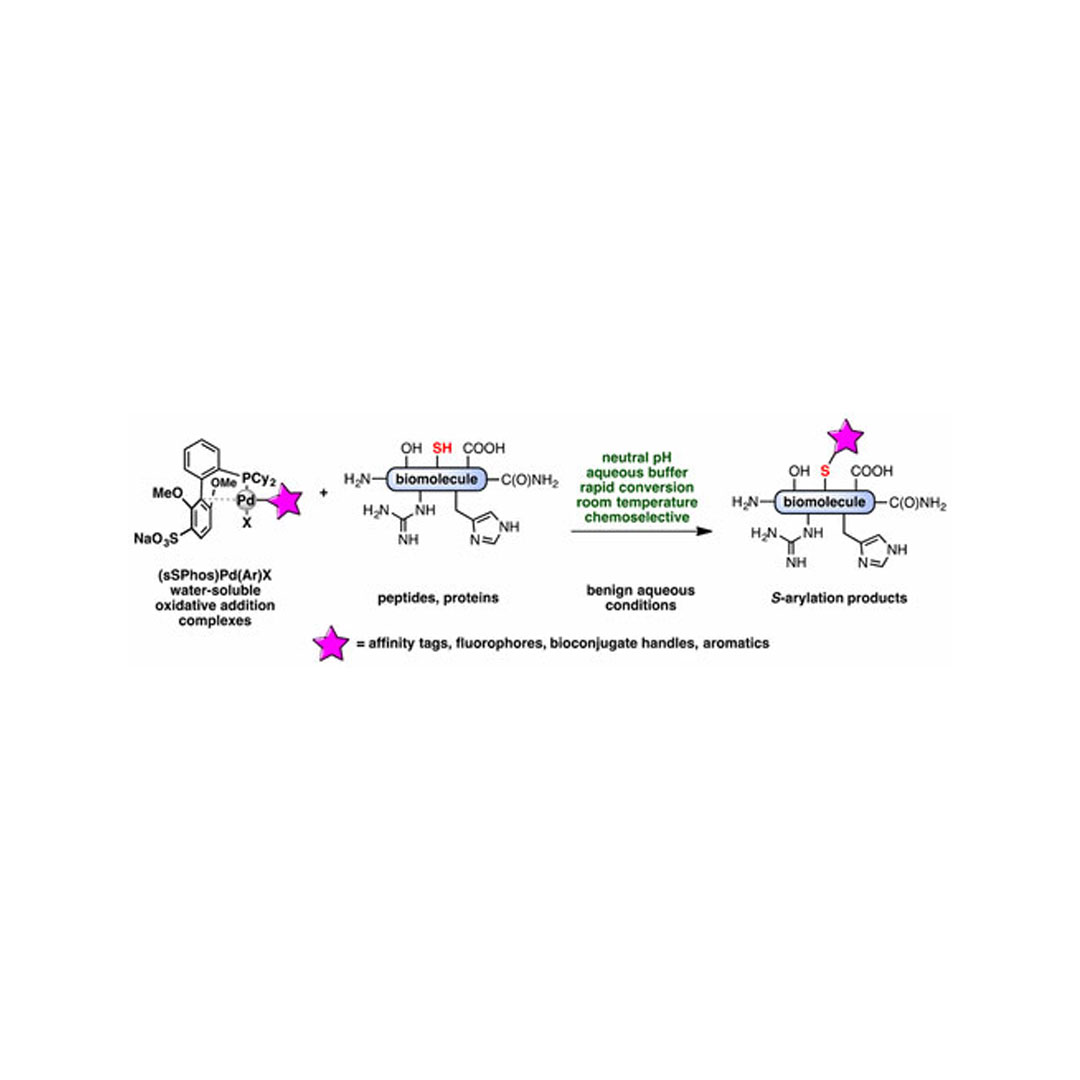
We report the use of a sulfonated biarylphosphine ligand (sSPhos) to promote the chemoselective modification of cysteine containing proteins and peptides with palladium reagents in aqueous medium. The use of sSPhos allowed for the isolation of several air-stable and water-soluble mono- and bis-palladium reagents, which were used in an improved protocol for the rapid S-arylation of cysteines under benign and physiologically relevant conditions. The cosolvent-free aqueous conditions were applied to the conjugation of a variety of biomolecules with affinity tags, heterocycles, fluorophores, and functional handles. Additionally, bis-palladium reagents were used to perform macrocyclization of peptides bearing two cysteine residues.
Pentelute Lab, MIT, Cambridge, Chemistry, Molecular biology, technology development, peptide, protein-based therapeutics, chemical Biology
17067
portfolio_page-template-default,single,single-portfolio_page,postid-17067,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Water-Soluble Palladium Reagents for Cysteine S-Arylation under Ambient Aqueous Conditions
Water-Soluble Palladium Reagents for Cysteine S-Arylation under Ambient Aqueous Conditions
Org. Lett., 2017, 19 (16), pp 4263–4266
DOI: 10.1021/acs.orglett.7b01911
Publication Date (Web): August 4, 2017
Anthony J. Rojas , Bradley L. Pentelute, and Stephen L. Buchwald
Abstract
We report the use of a sulfonated biarylphosphine ligand (sSPhos) to promote the chemoselective modification of cysteine containing proteins and peptides with palladium reagents in aqueous medium. The use of sSPhos allowed for the isolation of several air-stable and water-soluble mono- and bis-palladium reagents, which were used in an improved protocol for the rapid S-arylation of cysteines under benign and physiologically relevant conditions. The cosolvent-free aqueous conditions were applied to the conjugation of a variety of biomolecules with affinity tags, heterocycles, fluorophores, and functional handles. Additionally, bis-palladium reagents were used to perform macrocyclization of peptides bearing two cysteine residues.
Category
2017, Publications