Substrate Recognition of MARTX Ras/Rap1-Specific Endopeptidase
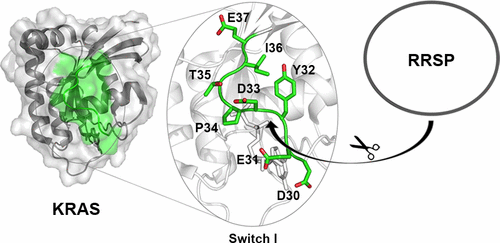
Ras/Rap1-specific endopeptidase (RRSP) is a cytotoxic effector domain of the multifunctional autoprocessing repeats-in-toxin (MARTX) toxin of highly virulent strains of Vibrio vulnificus. RRSP blocks RAS-MAPK kinase signaling by cleaving Ras and Rap1 within the switch I region between Y32 and D33. Although the RRSP processing site is highly conserved among small GTPases, only Ras and Rap1 have been identified as proteolytic substrates. Here we report that residues Y32 and D33 at the scissile bond play an important role in RRSP substrate recognition, while the nucleotide state of Ras has an only minimal effect. In addition, substrate specificity is generated by residues across the entire switch I region. Indeed, swapping the Ras switch I region into either RalA or RhoA, GTPases that are not recognized by RRSP, generated chimeras that are substrates of RRSP. However, a difference in the processing efficiency of Ras switch I in the context of Ras, RalA, or RhoA indicates that protein regions outside Ras switch I also contribute to efficient RRSP substrate recognition. Moreover, we show that synthetic peptides corresponding to the Ras and Rap1, but not RalA, switch I regions are cleaved by RRSP, demonstrating sequence-specific substrate recognition. In conclusion, this work demonstrates that the GTPase recognition of RRSP is independent of the nucleotide state and is mainly driven by the Ras and Rap1 switch I loop and also influenced by additional protein–protein interactions, increasing the substrate specificity of RRSP.
Pentelute Lab, MIT, Cambridge, Chemistry, Molecular biology, technology development, peptide, protein-based therapeutics, chemical Biology
17061
portfolio_page-template-default,single,single-portfolio_page,postid-17061,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Substrate Recognition of MARTX Ras/Rap1-Specific Endopeptidase
Substrate Recognition of MARTX Ras/Rap1-Specific Endopeptidase
Nature Biochemistry (2017)
DOI:10.1021/acs.biochem.7b00246
Publication Date (Web): May 1, 2017
Authors: Marco Biancucci, Amy E. Rabideau, Zeyu Lu, Alex R. Loftis, Bradley L. Pentelute, and Karla J. F. Satchell
Abstract
Ras/Rap1-specific endopeptidase (RRSP) is a cytotoxic effector domain of the multifunctional autoprocessing repeats-in-toxin (MARTX) toxin of highly virulent strains of Vibrio vulnificus. RRSP blocks RAS-MAPK kinase signaling by cleaving Ras and Rap1 within the switch I region between Y32 and D33. Although the RRSP processing site is highly conserved among small GTPases, only Ras and Rap1 have been identified as proteolytic substrates. Here we report that residues Y32 and D33 at the scissile bond play an important role in RRSP substrate recognition, while the nucleotide state of Ras has an only minimal effect. In addition, substrate specificity is generated by residues across the entire switch I region. Indeed, swapping the Ras switch I region into either RalA or RhoA, GTPases that are not recognized by RRSP, generated chimeras that are substrates of RRSP. However, a difference in the processing efficiency of Ras switch I in the context of Ras, RalA, or RhoA indicates that protein regions outside Ras switch I also contribute to efficient RRSP substrate recognition. Moreover, we show that synthetic peptides corresponding to the Ras and Rap1, but not RalA, switch I regions are cleaved by RRSP, demonstrating sequence-specific substrate recognition. In conclusion, this work demonstrates that the GTPase recognition of RRSP is independent of the nucleotide state and is mainly driven by the Ras and Rap1 switch I loop and also influenced by additional protein–protein interactions, increasing the substrate specificity of RRSP.
Category
2017, Publications