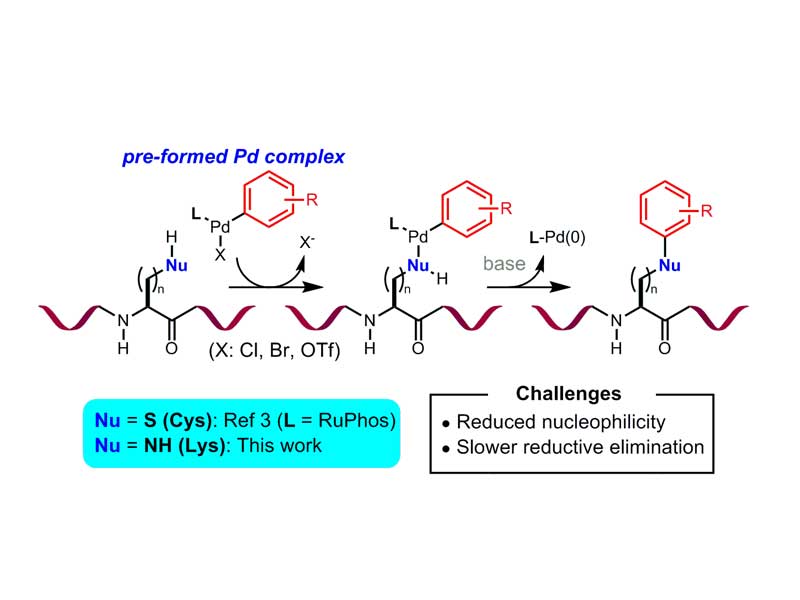
Palladium-Mediated Arylation of Lysine in Unprotected Peptides
A mild method for the arylation of lysine in an unprotected peptide is presented. In the presence of a preformed biarylphosphine-supported palladium(II)–aryl complex and a weak base, lysine amino groups underwent C−N bond formation at room temperature. The process generally exhibited high selectivity for lysine over other amino acids containing nucleophilic side chains and was applicable to the conjugation of a variety of organic compounds, including complex drug molecules, with an array of peptides. Finally, this method was also successfully applied to the formation of cyclic peptides by macrocyclization.
Pentelute Lab, MIT, Cambridge, Chemistry, Molecular biology, technology development, peptide, protein-based therapeutics, chemical Biology, Bradley Pentelute
16738
portfolio_page-template-default,single,single-portfolio_page,postid-16738,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Palladium-Mediated Arylation of Lysine in Unprotected Peptides
Palladium-Mediated Arylation of Lysine in Unprotected Peptides
DOI: 10.1002/anie.201611202
Published: 16 Feb 2017
Authors: Dr. Hong Geun Lee, Dr. Guillaume Lautrette, Prof. Bradley L. Pentelute, Prof. Stephen L. Buchwald
Abstract
A mild method for the arylation of lysine in an unprotected peptide is presented. In the presence of a preformed biarylphosphine-supported palladium(II)–aryl complex and a weak base, lysine amino groups underwent C−N bond formation at room temperature. The process generally exhibited high selectivity for lysine over other amino acids containing nucleophilic side chains and was applicable to the conjugation of a variety of organic compounds, including complex drug molecules, with an array of peptides. Finally, this method was also successfully applied to the formation of cyclic peptides by macrocyclization.
Category
2017, Publications