
Machine Learning To Predict Cell-Penetrating Peptides for Antisense Delivery
Machine Learning To Predict Cell-Penetrating Peptides for Antisense Delivery
ACS Cent. Sci., 2018, 4 (4), pp 512–520
DOI: 10.1021/acscentsci.8b00098
Publication Date (Web): April 5, 2018
Justin M. Wolfe , Colin M. Fadzen, Zi-Ning Choo, Rebecca L. Holden, Monica Yao, Gunnar J. Hanson, and Bradley L. Pentelute
Abstract
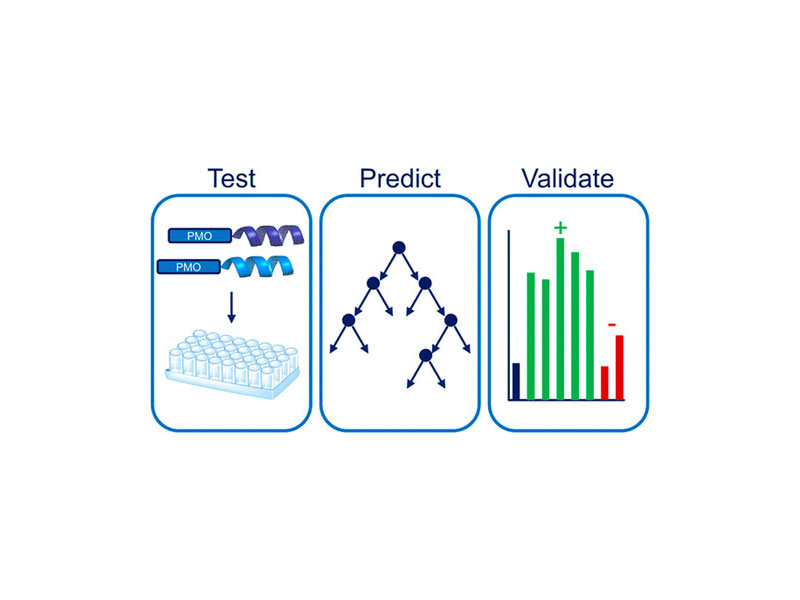
Cell-penetrating peptides (CPPs) can facilitate the intracellular delivery of large therapeutically relevant molecules, including proteins and oligonucleotides. Although hundreds of CPP sequences are described in the literature, predicting efficacious sequences remains difficult. Here, we focus specifically on predicting CPPs for the delivery of phosphorodiamidate morpholino oligonucleotides (PMOs), a compelling type of antisense therapeutic that has recently been FDA approved for the treatment of Duchenne muscular dystrophy. Using literature CPP sequences, 64 covalent PMO–CPP conjugates were synthesized and evaluated in a fluorescence-based reporter assay for PMO activity. Significant discrepancies were observed between the sequences that performed well in this assay and the sequences that performed well when conjugated to only a small-molecule fluorophore. As a result, we envisioned that our PMO–CPP library would be a useful training set for a computational model to predict CPPs for PMO delivery. We used the PMO activity data to fit a random decision forest classifier to predict whether or not covalent attachment of a given peptide would enhance PMO activity at least 3-fold. To validate the model experimentally, seven novel sequences were generated, synthesized, and tested in the fluorescence reporter assay. All computationally predicted positive sequences were positive in the assay, and one sequence performed better than 80% of the tested literature CPPs. These results demonstrate the power of machine learning algorithms to identify peptide sequences with particular functions and illustrate the importance of tailoring a CPP sequence to the cargo of interest.



