Heterochiral Knottin Protein: Folding and Solution Structure
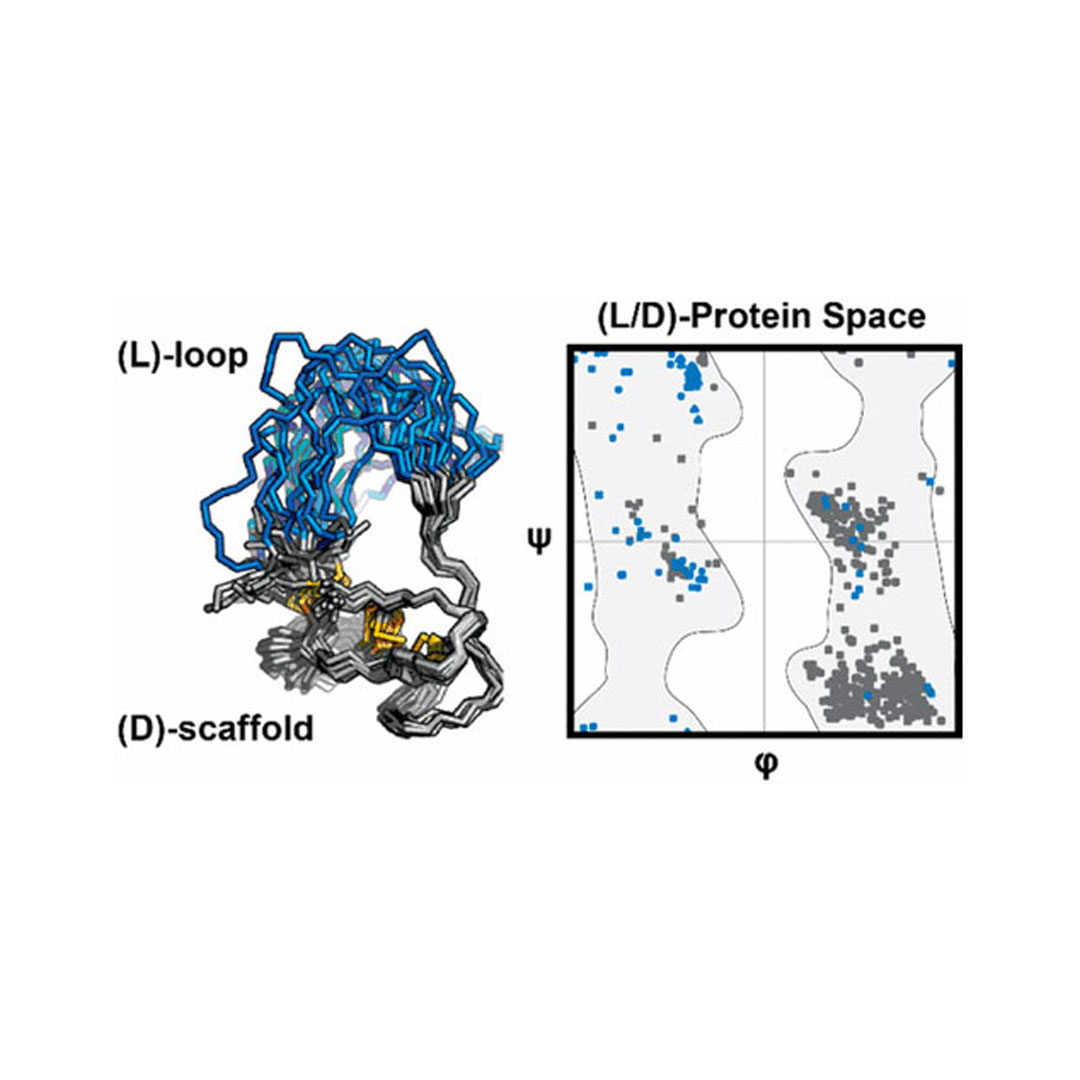
Homochirality is a general feature of biological macromolecules, and Nature includes few examples of heterochiral proteins. Herein, we report on the design, chemical synthesis, and structural characterization of heterochiral proteins possessing loops of amino acids of chirality opposite to that of the rest of a protein scaffold. Using the protein Ecballium elaterium trypsin inhibitor II, we discover that selective β-alanine substitution favors the efficient folding of our heterochiral constructs. Solution nuclear magnetic resonance spectroscopy of one such heterochiral protein reveals a homogeneous global fold. Additionally, steered molecular dynamics simulation indicate β-alanine reduces the free energy required to fold the protein. We also find these heterochiral proteins to be more resistant to proteolysis than homochiral l-proteins. This work informs the design of heterochiral protein architectures containing stretches of both d- and l-amino acids.
Pentelute Lab, MIT, Cambridge, Chemistry, Molecular biology, technology development, peptide, protein-based therapeutics, chemical Biology
17071
portfolio_page-template-default,single,single-portfolio_page,postid-17071,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Heterochiral Knottin Protein: Folding and Solution Structure
Heterochiral Knottin Protein: Folding and Solution Structure
Biochemistry, 2017, 56 (43), pp 5720–5725
DOI: 10.1021/acs.biochem.7b00722
Publication Date (Web): September 27, 2017
Surin K. Mong, Frank V. Cochran, Hongtao Yu, Zachary Graziano, Yu-Shan Lin , Jennifer R. Cochran, and Bradley L. Pentelute
Abstract
Homochirality is a general feature of biological macromolecules, and Nature includes few examples of heterochiral proteins. Herein, we report on the design, chemical synthesis, and structural characterization of heterochiral proteins possessing loops of amino acids of chirality opposite to that of the rest of a protein scaffold. Using the protein Ecballium elaterium trypsin inhibitor II, we discover that selective β-alanine substitution favors the efficient folding of our heterochiral constructs. Solution nuclear magnetic resonance spectroscopy of one such heterochiral protein reveals a homogeneous global fold. Additionally, steered molecular dynamics simulation indicate β-alanine reduces the free energy required to fold the protein. We also find these heterochiral proteins to be more resistant to proteolysis than homochiral l-proteins. This work informs the design of heterochiral protein architectures containing stretches of both d- and l-amino acids.
Category
2017, Publications