
Evidence for a Common Mechanism of SIRT1 Regulation by Allosteric Activators
Evidence for a Common Mechanism of SIRT1 Regulation by Allosteric Activators
Hubbard, Gomes, Dai, Li, Case, Considine, Riera, Lee, Lamming, Pentelute, Schuman, Stevens, Ling, Armour, Michan, Zhao, Jiang, Sweitzer, Blum, Disch, Ng, Howitz, Rolo, Hamuro, Moss, Perni, Ellis, Vlasuk, Sinclair, Science, 2013, 339, 1216-1219. Highlighted by ScienceDaily, C&EN News and F1000Prime.
Published
Online March, 8 2013
Abstract
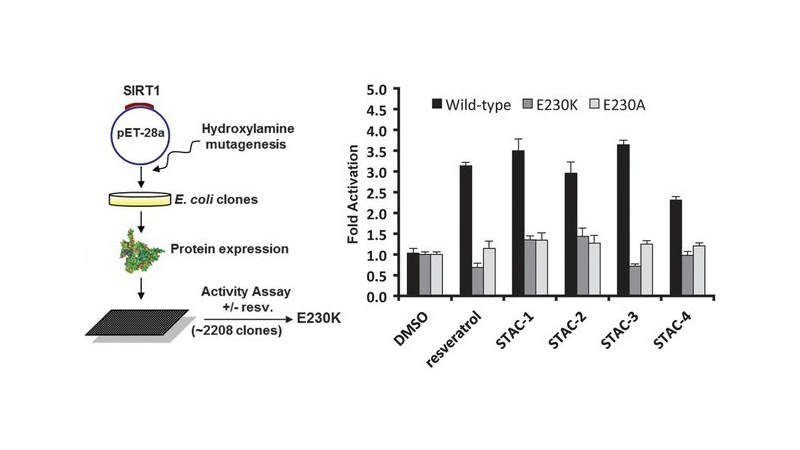
A molecule that treats multiple age-related diseases would have a major impact on global health and economics. The SIRT1 deacetylase has drawn attention in this regard as a target for drug design. Yet controversy exists around the mechanism of sirtuin-activating compounds (STACs). We found that specific hydrophobic motifs found in SIRT1 substrates such as PGC-1α and FOXO3a facilitate SIRT1 activation by STACs. A single amino acid in SIRT1, Glu(230), located in a structured N-terminal domain, was critical for activation by all previously reported STAC scaffolds and a new class of chemically distinct activators. In primary cells reconstituted with activation-defective SIRT1, the metabolic effects of STACs were blocked. Thus, SIRT1 can be directly activated through an allosteric mechanism common to chemically diverse STACs.



