
Enzymatic “Click” Ligation: Selective Cysteine Modification in Polypeptides Enabled by Promiscuous Glutathione S-Transferase
Enzymatic “Click” Ligation: Selective Cysteine Modification in Polypeptides Enabled by Promiscuous Glutathione S-Transferase
Zhang C, Spokoyny AM, Zou Y, Simon MD, Pentelute BL., Angew Chem Int Ed Engl. 2013 Dec 23;52(52):14001-5.
Published
Online November 12, 2013
Abstract
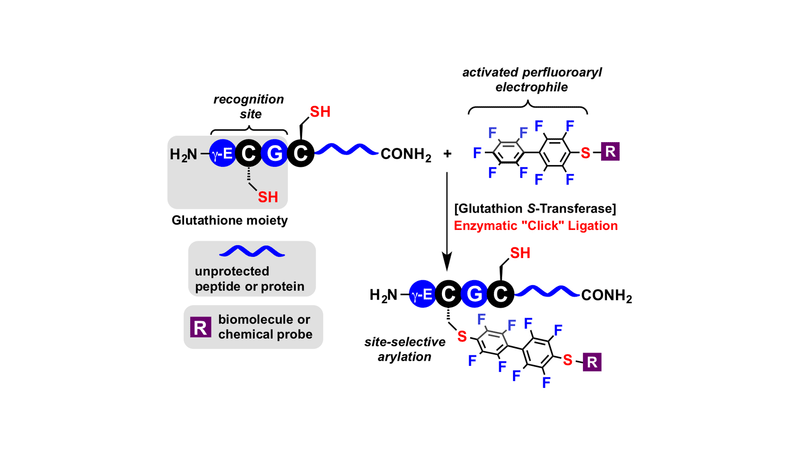
Singled out for special treatment: Naturally occurring glutathione S-transferase (GST) was used to catalyze an efficient “click” ligation between polypeptides with an N-terminal glutathione sequence and biomolecules or chemical probes containing perfluorinated aromatic groups (see scheme). The site-specific modification of one cysteine residue was possible in the presence of other unprotected cysteine residues and reactive functional groups.



