
Discovery of a 29-Amino-Acid Reactive Abiotic Peptide for Selective Cysteine Arylation
Discovery of a 29-Amino-Acid Reactive Abiotic Peptide for Selective Cysteine Arylation
ACS Chem. Biol., 2018, 13 (3), pp 527–532
DOI: 10.1021/acschembio.7b00520
Publication Date (Web): December 28, 2017
Ethan D. Evans and Bradley L. Pentelute*
Abstract
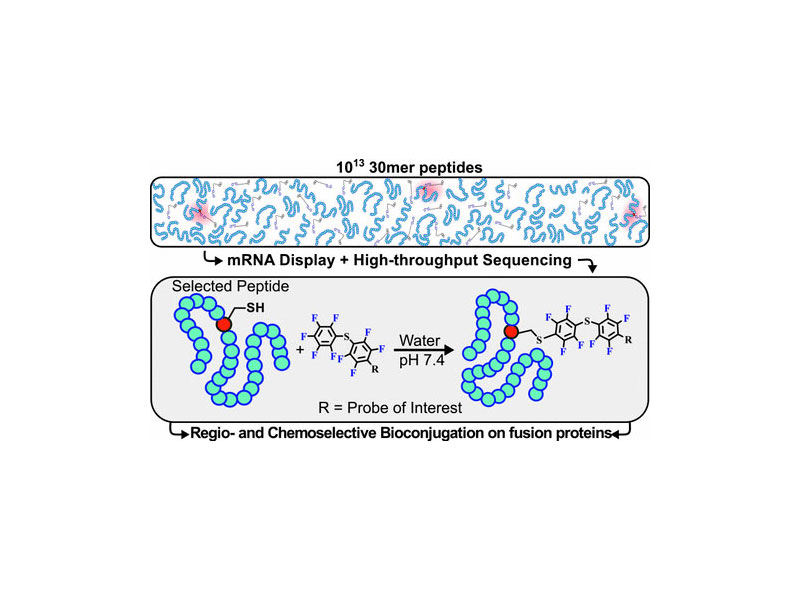
The regio- and chemoselective modification of proteins or peptides with chemical reagents is often challenging. One approach to overcome this problem involves identifying abiotic polypeptide sequences that react with specific small molecules. Toward this goal, we profiled ∼5 × 1013 randomized 30-mer peptides using mRNA display and high-throughput sequencing in search of polypeptides that can undergo cysteine arylation with a water-soluble perfluoroarene. Within this vast chemical space, we discovered a cysteine-containing sequence with a second-order rate constant of 0.29 M–1 s–1 for arylation. An N- and C-terminal truncation reduced the reaction rate, as did the addition of denaturants. When the reactive peptide was covalently fused to the enzyme Sortase A, we observed regiospecific arylation at a single cysteine site, leaving the enzyme’s active site cysteine unchanged. Taken together, these results demonstrate that long polypeptides of defined sequence, when matched with the appropriate reactive group, can be used for selective arylation of cysteine in water.



