Amide-forming chemical ligation via O-acyl hydroxamic acids
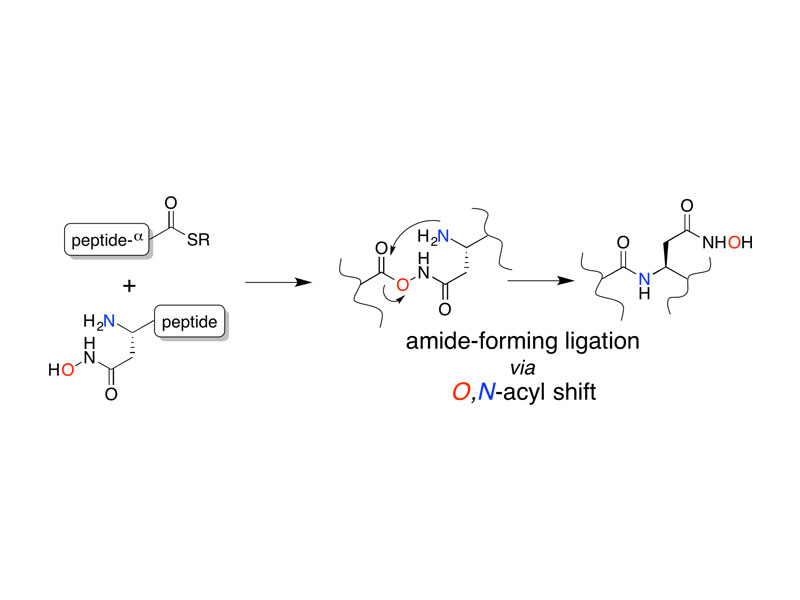
The facile rearrangement of “S-acyl isopeptides” to native peptide bonds via S,N-acyl shift is central to the success of native chemical ligation, the widely used approach for protein total synthesis. Proximity-driven amide bond formation via acyl transfer reactions in other contexts has proven generally less effective. Here, we show that under neutral aqueous conditions, “O-acyl isopeptides” derived from hydroxy-asparagine [aspartic acid-β-hydroxamic acid; Asp(β-HA)] rearrange to form native peptide bonds via an O,N-acyl shift. This process constitutes a rare example of an O,N-acyl shift that proceeds rapidly across a medium-size ring (t1/2 ∼ 15 min), and takes place in water with minimal interference from hydrolysis. In contrast to serine/threonine or tyrosine, which form O-acyl isopeptides only by the use of highly activated acyl donors and appropriate protecting groups in organic solvent, Asp(β-HA) is sufficiently reactive to form O-acyl isopeptides by treatment with an unprotected peptide-αthioester, at low mM concentration, in water. These findings were applied to an acyl transfer-based chemical ligation strategy, in which an unprotected N-terminal Asp(β-HA)-peptide and peptide-αthioester react under aqueous conditions to give a ligation product ultimately linked by a native peptide bond.
Amide-forming chemical ligation via O-acyl hydroxamic acids
17247
portfolio_page-template-default,single,single-portfolio_page,postid-17247,bridge-core-3.0.1,qode-page-transition-enabled,ajax_fade,page_not_loaded,,paspartu_enabled,paspartu_on_top_fixed,paspartu_on_bottom_fixed,qode_grid_1200,qode_popup_menu_push_text_top,qode-theme-ver-28.7,qode-theme-bridge,disabled_footer_top,wpb-js-composer js-comp-ver-6.8.0,vc_responsive
Amide-forming chemical ligation via O-acyl hydroxamic acids
Amide-forming chemical ligation via O-acyl hydroxamic acids
PNAS April 10, 2018. 115 (15) 3752-3757; published ahead of print March 26, 2018. https://doi.org/10.1073/pnas.1718356115
Daniel L. Dunkelmann, Yuki Hirata, Kyle A. Totaro, Daniel T. Cohen, Chi Zhang, Zachary P. Gates, and Bradley L. Pentelute
Abstract
The facile rearrangement of “S-acyl isopeptides” to native peptide bonds via S,N-acyl shift is central to the success of native chemical ligation, the widely used approach for protein total synthesis. Proximity-driven amide bond formation via acyl transfer reactions in other contexts has proven generally less effective. Here, we show that under neutral aqueous conditions, “O-acyl isopeptides” derived from hydroxy-asparagine [aspartic acid-β-hydroxamic acid; Asp(β-HA)] rearrange to form native peptide bonds via an O,N-acyl shift. This process constitutes a rare example of an O,N-acyl shift that proceeds rapidly across a medium-size ring (t1/2 ∼ 15 min), and takes place in water with minimal interference from hydrolysis. In contrast to serine/threonine or tyrosine, which form O-acyl isopeptides only by the use of highly activated acyl donors and appropriate protecting groups in organic solvent, Asp(β-HA) is sufficiently reactive to form O-acyl isopeptides by treatment with an unprotected peptide-αthioester, at low mM concentration, in water. These findings were applied to an acyl transfer-based chemical ligation strategy, in which an unprotected N-terminal Asp(β-HA)-peptide and peptide-αthioester react under aqueous conditions to give a ligation product ultimately linked by a native peptide bond.
Category
2018, Publications