
A Perfluoroaryl-Cysteine Chemistry Approach to Unprotected Peptide Stapling
A Perfluoroaryl-Cysteine Chemistry Approach to Unprotected Peptide Stapling
Spokoyny, Zou, Ling, Yo, Lin, Pentelute, J. Am. Chen. Soc. 2013, 135, 5946-5949
Published
Online April 5, 2013
Abstract
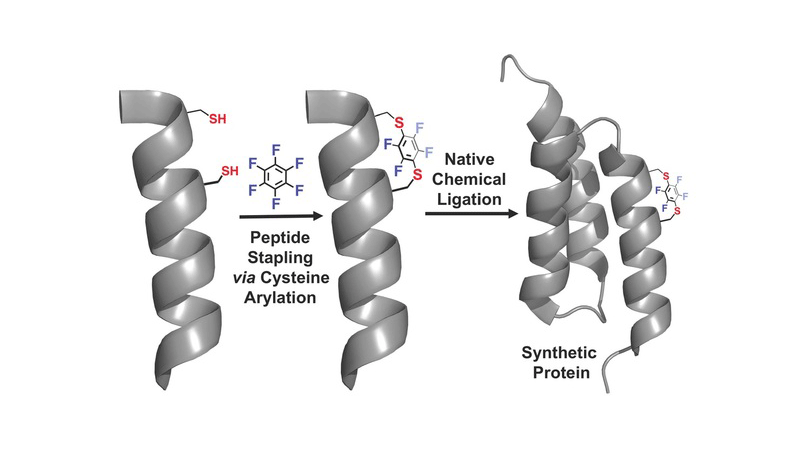
We report the discovery of a facile transformation between perfluoroaromatic molecules and a cysteine thiolate, which is arylated at room temperature. This new approach enabled us to selectively modify cysteine residues in unprotected peptides, providing access to variants containing rigid perfluoroaromatic staples. This stapling modification performed on a peptide sequence designed to bind the C-terminal domain of an HIV-1 capsid assembly polyprotein (C-CA) showed enhancement in binding, cell permeability, and proteolytic stability properties, as compared to the unstapled analog. Importantly, chemical stability of the formed staples allowed us to use this motif in the native chemical ligation-mediated synthesis of a small protein affibody that is capable of binding the human epidermal growth factor 2 receptor.



